Abstract
BACKGROUND: Chronic lymphocytic leukemia (CLL) is one of the most common lymphoid malignancies, accounting for ~11% of all hematologic neoplasms. Over the last 15 years, a series of phase 3 trials have established that chemoimmunotherapy (CIT) with fludarabine, cyclophosphamide, and rituximab (FCR) improves both progression free survival (PFS) and overall survival (OS) compared with chemotherapy alone. FCR is the gold standard for young fit patients with treatment naïve CLL.
In parallel with the advances in CIT, a profound increase in the understanding of CLL B-cell biology led to new therapeutic approaches.1 Among these, ibrutinib (an irreversible inhibitor of Bruton's Tyrosine Kinase [BTK]) has had the largest impact on clinical practice to date. Initial trials of ibrutinib demonstrated robust and durable efficacy in patients with relapsed/refractory disease. Subsequent phase 3 trials showed improved PFS and OS with ibrutinib relative to chlorambucil in previously untreated, older CLL patients. Despite these advances, the efficacy of ibrutinib as a first-line treatment for younger CLL patients (i.e. <70) relative to the most efficacious CIT regimens, such as FCR, is unknown.
METHODS: Eligible patients were treatment-naive individuals with CLL who were age <70 and required therapy. Patients with deletion 17p- were excluded from participating given the poor response of these patients to FCR therapy. Participants were randomly assigned in a 2:1 ratio to receive ibrutinib (420 mg/day until disease progression) and rituximab (50 mg/m2 on day 1 of cycle 2; 325 mg/m2 on day 2 of cycle 2; 500 mg/m2 on day 1 of cycles 3-7) or six courses of intravenous fludarabine (25 mg/m2 ) and cyclophosphamide (250 mg/m2) days 1-3 with rituximab (50 mg/m2 on day 1 of cycle 1; 325 mg/m2 on day 2 of cycle 1; 500 mg/m2 on day 1 of cycles 2-6) every 28-days. The planned accrual was 519 patients.
Hematologic toxicity was graded according to the 2008 IWCLL Working Group scale. All other adverse events were graded according to the NCI Common Toxicity Criteria (version 4).
The primary endpoint was PFS with a secondary endpoint of overall survival (OS). Analysis was by intention to treat. The first planed interim analysis for PFS was scheduled to occur 24-27 months after full accrual with the first interim analysis for OS scheduled to occur if the boundary for PFS was crossed. The primary analysis was a stratified logrank test applied to all patients as randomized. Treatment effect p-values are one-sided.
The study was approved by the Central Institutional Review Board for the National Cancer Institute, conducted in accordance with the principles of the Declaration of Helsinki, and registered with ClinicalTrials.gov (NCT02048813).
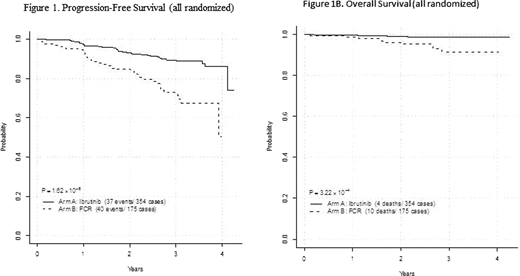
RESULTS: A total of 529 patients were accrued between January 31, 2014 and June 9, 2016. 354 patients were assigned to ibrutinib and rituximab (IR) and 175 to FCR. Nineteen patients did not start protocol therapy. The first interim analysis was performed September 2018. With median follow-up of 33.4 months, we observed 77 PFS events and 14 deaths. The hazard ratio (HR) for PFS favored IR over FCR (HR=0.352; 95% CI 0.223-0.558; p<0.0001) which crossed the pre specified boundary. The HR for OS also favored the IR arm (HR=0.168, 95% CI 0.053-0.538; p=0.0003, pre-specified boundary for superiority p=0.0005). Kaplan-Meier estimates for PFS and OS are shown in Figure 1A and 1B.
In subgroup analysis for PFS, IR was superior to FCR independent of age, sex, performance status, disease stage or the presence/absence of del11q23. With current follow-up, IR was also superior to FCR for IGHV unmutated patients (HR=0.262; 95% CI 0.137-0.498; p<0.0001) but not IGHV mutated patients (HR=0.435; 95% CI 0.140-0.1350; p=0.07).
Grade 3 and 4 treatment-related adverse events were observed in 58% of IR and 72% of FCR treated patients (p=0.0042). Specifically, FCR was more frequently associated with grade 3 and 4 neutropenia (FCR: 69 [44%] of 158 vs. IR: 80 [23%] of 352; p<0·0001) and infectious complications (FCR: 28/158 [17.7%] vs. IR: 25/352 [7.1%]; p<0.0001).
CONCLUSIONS: The combination of ibrutinib and rituximab provides superior PFS and OS relative to FCR for patients with previously untreated CLL age <70. These findings have immediate practice changing implications and establish ibrutinib-based therapy as the most efficacious first-line therapy for patients with CLL.
Shanafelt:Mayo Clinic: Patents & Royalties: Physician Well-being Index, Medical Student Well-being Index, Well-being index; Celgene: Research Funding; GlaxoSmithKline: Research Funding; Genentech: Research Funding; Abbvie: Research Funding; Pharmacyclics: Research Funding; Janssen: Research Funding. Kay:Janssen: Membership on an entity's Board of Directors or advisory committees; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Cytomx Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Infinity Pharm: Membership on an entity's Board of Directors or advisory committees. O'Brien:Amgen: Consultancy; Astellas: Consultancy; Celgene: Consultancy; GlaxoSmithKline: Consultancy; Janssen: Consultancy; Aptose Biosciences Inc.: Consultancy; Vaniam Group LLC: Consultancy; Abbvie: Consultancy; Alexion: Consultancy; Kite Pharma: Research Funding; Regeneron: Research Funding; Acerta: Research Funding; Gilead: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding. Barrientos:Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; astra Zeneca: Membership on an entity's Board of Directors or advisory committees. Erba:Seattle Genetics: Consultancy, Research Funding; Pfizer: Consultancy, Other: grant; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Other: grant; Pfizer: Consultancy, Other: grant; Incyte: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Astellas: Research Funding; Janssen: Research Funding; Janssen: Research Funding; Amgen: Research Funding; Amgen: Research Funding; Novartis: Consultancy, Speakers Bureau; Astellas: Research Funding; Incyte: Consultancy, Speakers Bureau; Astellas: Research Funding; Pfizer: Consultancy, Other: grant; Novartis: Consultancy, Speakers Bureau; Juno: Research Funding; Juno: Research Funding; Novartis: Consultancy, Speakers Bureau; MacroGenics: Consultancy; MacroGenics: Consultancy; Amgen: Research Funding; Takeda/Millenium: Research Funding; Amgen: Research Funding; Takeda/Millenium: Research Funding; Seattle Genetics: Consultancy, Research Funding; Jazz: Consultancy, Speakers Bureau; Immunogen: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Jazz: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy, Speakers Bureau; Takeda/Millenium: Research Funding; Immunogen: Consultancy, Research Funding; Takeda/Millenium: Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Celgene: Consultancy, Speakers Bureau; MacroGenics: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; MacroGenics: Consultancy; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Daiichi Sankyo: Consultancy, Research Funding; Juno: Research Funding; Juno: Research Funding; Agios: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Immunogen: Consultancy, Research Funding; Jazz: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Immunogen: Consultancy, Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Celgene: Consultancy, Speakers Bureau; Daiichi Sankyo: Consultancy, Research Funding; Glycomimetics: Consultancy, Other: Chair, Data and Safety Monitoring Committee; Daiichi Sankyo: Consultancy, Research Funding. Stone:Juno: Consultancy; Macrogenics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; FujiFilm: Consultancy; Sumitomo: Consultancy; Ono/Theradex: Consultancy; Otzuka/Astex: Consultancy; Pfizer: Consultancy; Roche: Consultancy; AbbVie: Consultancy, Research Funding; Actinium: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Argenx: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; Celator / Jazz: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cornerstone: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Tallman:ADC Therapeutics: Research Funding; Daiichi-Sankyo: Other: Advisory board; BioSight: Other: Advisory board; AbbVie: Research Funding; Cellerant: Research Funding; AROG: Research Funding; Orsenix: Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal